Researcher in Residence: Advancing Phage Therapy in the UK
As antimicrobial resistance continues to rise, the exploration of alternative therapies becomes increasingly crucial. Bacteriophage (phage) therapy, harnessing viruses to combat bacterial infections, emerges as a promising solution. Recognising its potential, the UK Parliament’s Science and Technology Committee initiated an inquiry into the safety and effectiveness of phages. Another person who has recognised its potential is Liberty Duignan from the University of Liverpool who has been awarded a grant from the Innovation Launchpad Network+ to advance her research on this area in collaboration with Medicines Discovery Catapult and High Value Manufacturing Catapult’s CPI.
Recent clinical data revealed promising results, with 87% of patients achieving bacterial eradication, prompting countries like Belgium and Australia to adopt phage therapy clinically. However, in the UK, regulatory classification as a biological medicine and lack of a regulatory framework which has hindered their manufacture and clinical use, leading to compassionate use cases sourced from overseas.
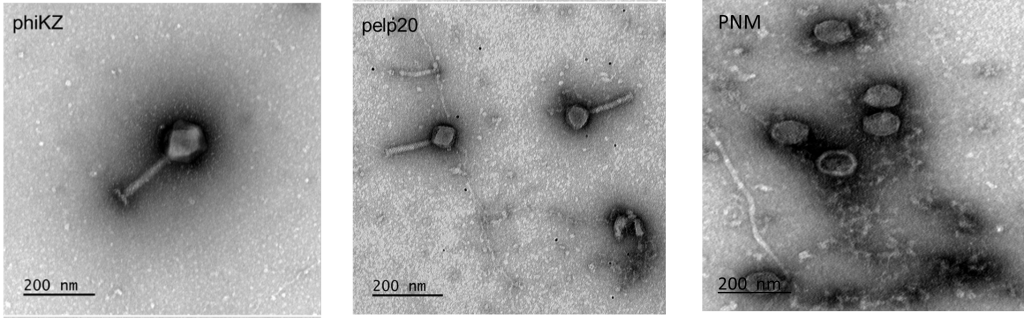
These three genetically and morphologically distinct Pseudomonas phages will be used determine the impact of diversity on manufacturing processes.
At the University of Liverpool, researchers have been studying well characterised phages targeting Pseudomonas aeruginosa, a priority pathogen according to the World Health Organization. This project, in collaboration with the Centre for Process Innovation (CPI) and the Medicines Discovery Catapult (MDC), aims to overcome manufacturing barriers in the UK by fostering knowledge exchange and expertise.
Currently, the UK lacks a robust phage industry. Through this work, the country could bolster its resilience against antibiotic-resistant bacteria and pave the way for the emergence of phage-based industries. By establishing expertise and facilities for phage manufacture, the project aims to propel the UK to the forefront of phage therapy research and development.
Libby said of the project: “There is so much research into phage therapy in the UK, against many different bacterial species and within a large number of infection niches, the two things that is holding this extremely useful therapeutic back is the lack of regulatory framework and a manufacturing process to GMP. My working with CPI and MDC we will be paving the way for phage manufacturing and enabling the start of phage therapy clinical trials with are critical to getting them to market.”



